Facts About Carbon: Welcome to FactsCrush.Com. In this article we will know some facts related to Carbon. We have done a lot of research on this topic. We hope that you will definitely get the information you need related to Carbon here.
The element carbon is an important element of the periodic table. The molecular number of carbon is 6. It is denoted by "C". Carbon is a very important element in chemistry and biochemistry. Carbon content is the highest among all other substances except water in the human body. Organisms are made up of different types of organic compounds.
Fun Facts About Carbon for Kids
- The symbol for carbon is C.
- Carbon has 15 known isotopes.
- The atomic number for carbon is 6.
- Carbon is a solid at room temperature.
- Carbon makes up 20% of the weight of the human body.
- Carbon is a chemical element on the periodic table.
- The standard atomic weight for carbon is 12.0107 u.
- The melting point for carbon is 6,422 °F (3,550 °C).
- The boiling point for carbon is 6,917 °F (3,825 °C).
- About 20% of the weight of living organisms is carbon.
- Carbon is the 15th most abundant element found on Earth.
- Graphene is the thinnest, strongest material ever known.
- More compounds are known which contain carbon than don’t.
- Carbon is the fourth most abundant element in the universe.
- Carbon is based on the Latin word carbo, which means “coal”.
- Carbon is the 4th most abundant element found in the universe.
- Diamonds are made of carbon and form deep in the Earth’s mantle.
- Carbon is a group 14 chemical element, which is the carbon group.
- Carbon is in the reactive nonmetal element category on the periodic table.
- Carbon was identified as an element in 1789, by French chemist Antoine Lavoisier.
- Carbon is a period 2 chemical element, which is the second row on the periodic table.
Scientific Facts About Carbon
- Despite its high abundance, we owe Carbon’s existence to an unlikely set of circumstances
- Coal is made of carbon and various other elements (hydrogen, nitrogen, oxygen and sulfur).
- Carbon undergoes nuclear fusion reactions in heavy stars to make neon, magnesium and oxygen.
- Carbon monoxide is a byproduct of burning fossil fuels, this odorless gas is deadly to humans.
- Carbon dioxide is a byproduct exhaled by humans and makes up about 4% of the Earth’s atmosphere.
- Carbon can be used to determine the age of organic material using a process called carbon dating.
- The carbon atoms in your body were all once part of the carbon dioxide fraction of the atmosphere.
- Graphene is made of 2-dimensional atomic crystals, the first time such structures have ever been seen.
- Elemental carbon can take the form of one of the hardest substances (diamond) or one of the softest (graphite).
- The graphite in a typical mechanical pencil has a diameter of 0.7 mm. This is equal to 2 million layers of graphene.
- The origin of the name "carbon" comes from the Latin word carbo, for charcoal. The German and French words for charcoal are similar.
- Carbon is made within stars when they burn helium in nuclear fusion reactions. Carbon is part of the ‘ash’ formed by helium burning.
Wierd Facts About Carbon
- Inorganic carbon sources include carbon dioxide, limestone, and dolomite. Organic sources include coal, oil, peat, and methane clathrates.
- Carbon black was the first pigment used for tattooing. Ötzi the Iceman has carbon tattoos that endured through his life and are still visible 5200 years later.
- Car tires are black because they are about 30% carbon black, which is added to rubber to strengthen it. The carbon black also helps protect against UV damage to the tires.(8)
- Carbon usually has a valence of +4, which means each carbon atom can form covalent bonds with four other atoms. The +2 oxidation state is also seen in compounds such as carbon monoxide.
- Carbon is the fourth most abundant element in the universe (hydrogen, helium, and oxygen are found in higher amounts, by mass). It is the 15th most abundant element in the Earth's crust.
- Diamond is an excellent abrasive because it is the hardest common material and it also has the highest thermal conductivity. It can grind down any substance, while the heat generated by friction is swiftly conducted away.
- Carbon is a nonmetal that can bond with itself and many other chemical elements, forming over ten million compounds. Because it forms more compounds than any other element, it is sometimes called the "King of the Elements."
- Carbon is the basis for organic chemistry, as it occurs in all living organisms. The simplest organic molecules consist of carbon chemically bonded to hydrogen. Many other common organics also include oxygen, nitrogen, phosphorus, and sulfur.
Mind-Blowing Facts About Carbon
- Pure carbon is considered non-toxic, although inhalation of fine particles, such as soot, can damage lung tissue. Graphite and charcoal are considered safe enough to eat. While non-toxic to humans, carbon nanoparticles are deadly to fruit flies.
- Carbon has the highest melting/sublimation point of the elements. The melting point of diamond is ~3550°C, with the sublimation point of carbon around 3800°C. If you baked a diamond in an oven or cooked it in a frying pan, it would survive unscathed.
- Carbon compounds have limitless uses. In its elemental form, diamond is a gemstone and used for drilling/cutting; graphite is used in pencils, as a lubricant, and to protect against rust; while charcoal is used to remove toxins, tastes, and odors. The isotope Carbon-14 is used in radiocarbon dating.
- Carbon is made in the interiors of stars, although it was not produced in the Big Bang. Carbon is made in giant and supergiant stars via the triple-alpha process. In this process, three helium nuclei fuse. When a massive star turns into a supernova, carbon scatters and can be incorporated into next-generation stars and planets.
- Three isotopes of carbon occur naturally. Carbon-12 and carbon-13 are stable, while carbon-14 is radioactive, with a half-life of around 5730 years. Carbon-14 is formed in the upper atmosphere when cosmic rays interact with nitrogen. While carbon-14 occurs in the atmosphere and living organisms, it is almost completely absent from rocks. There are 15 known carbon isotopes.
- The amount of carbon on Earth is fairly constant. It is transformed from one form to another via the carbon cycle. In the carbon cycle, photosynthetic plants take carbon from air or seawater and convert it into glucose and other organic compounds via the Calvin cycle of photosynthesis. Animals eat some of the biomass and exhale carbon dioxide, returning carbon to the atmosphere.
- Pure carbon exists free in nature and has been known since prehistoric time. While most elements known since ancient time only exist in one allotrope, pure carbon forms graphite, diamond, and amorphous carbon (soot). The forms look very different from each other and display dissimilar properties. For example, graphite is an electrical conductor while diamond is an insulator. Other forms of carbon include fullerenes, graphene, carbon nanofoam, glassy carbon, and Q-carbon (which is magnetic and fluorescent).
Friends, hope you liked this post on Fun Facts About Carbon for Kids. If you liked this post, then you must share it with your friends and Subscribe to us to get updates from our blog. Friends, If you liked our site FactsCrush.Com, then you should Bookmark it as well.




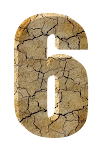









0 Comments